COVID-19 Rapid Test Kit for Detection of
IgG and IgM Antibodies
Now Available for Supply
This Antibody Based Rapid Test Kit sits under the EU Directives for In Vitro Diagnostic Medical Devices Directive 98/79/EC.
It performs qualitatively to detect IgG and IgM antibodies to SARS-CoV-2 in human whole blood, serum or plasma samples. The kit does not contain any invasive components such as needles or lancets to draw the human whole blood, serum or plasma samples which the healthcare professional need to provide to perform the tests.
Once the human whole blood, serum or plasma samples are placed in the clearly marked hole in each of the two cartridges designed to detect each IgG and IgM antibodies. Two separate test cartridges preferred over one for clear separate results.
Results for each test will be seen within 15 to 20 minutes. The diagrams below illustrate:
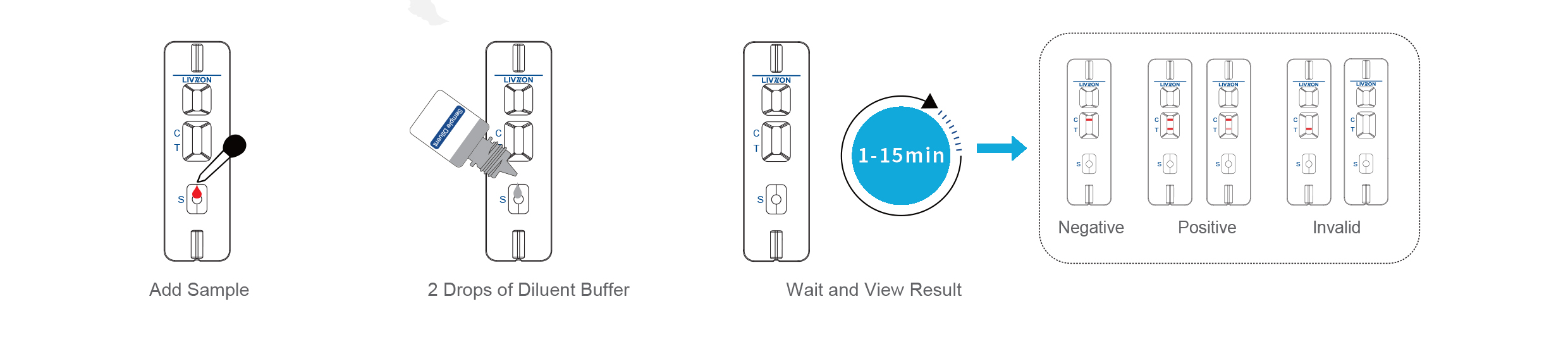
A detailed user leaflet comes in each box. English language only. Ordering will be by number of tests required, each test being two cartridges one for each antibody.
NOTE: Our IgM and IgG Rapid Test Kit is an Antibody test to confirm a diagnosis of COVID-19. It does not test Antigens. The test kit has EU CE Mark registration.
Click below to download our Technical Data Sheet and Spec Sheet

 IPG Pharma Group Brochure 3 FINAL
IPG Pharma Group Brochure 3 FINAL